Kai Van Beek 10 min read
Application Note: BSE Contrast
Contributors
Kai Van Beek
Subscribe to our newsletter
Particles have a profound impact on society, particularly within the fields of materials science and environmental sciences. In materials science, the study of particles has revolutionized the development of new materials with enhanced properties. Tailoring nano- and microparticles to exhibit unique characteristics such as high strength, conductivity, or catalytic activity has led to advancements in areas such as renewable energy, additive manufacturing, powered metals, energy storage, and more.
Additionally, the manipulation of particles at the atomic and molecular level has enabled the creation of innovative materials with applications in electronics, optoelectronics, and biomaterials. In environmental sciences, particles play a crucial role in understanding and mitigating pollution and its impact on ecosystems and human health. Particulate matter, including airborne pollutants and microplastics, is of great concern due to its ability to be transported over long distances and its potential to cause respiratory problems, cardiovascular diseases, and other adverse health effects.
Furthermore, the study of particles in environmental sciences extends to understanding natural processes such as sedimentation, erosion, and the transport of nutrients and contaminants in ecosystems. In conclusion, particles have a profound impact on society, they drive advancements in materials design, renewable energy, and electronics, while also influencing pollution control strategies, environmental policy, and the protection of ecosystems and human health.
Understanding and harnessing the behavior of particles is crucial for sustainable development, addressing environmental challenges, and building a healthier and more resilient future. Scanning electron microscopes are one of the most powerful analytical tools to study the size, shape, and morphology of particles, but this is not without its pitfalls. RJ Lee Group uses a variety of techniques for comprehensive particle characterization and RJ Lee Group’s IntelliSEM provides the platform to conduct and organize particle characterization studies.
Particle Analysis with Scanning Electron Microscope
Scanning electron microscopes are one of the most powerful analytical techniques to study particles. Not only because of the high magnifications but also because many scanning electron microscopes are equipped with backscatter electron detector(s). The backscatter electron detector provides a first clue on the chemistry of any particles as low atomic materials have a low backscatter coefficient and high atomic numbers have a high backscatter coefficient. (see figure 1)
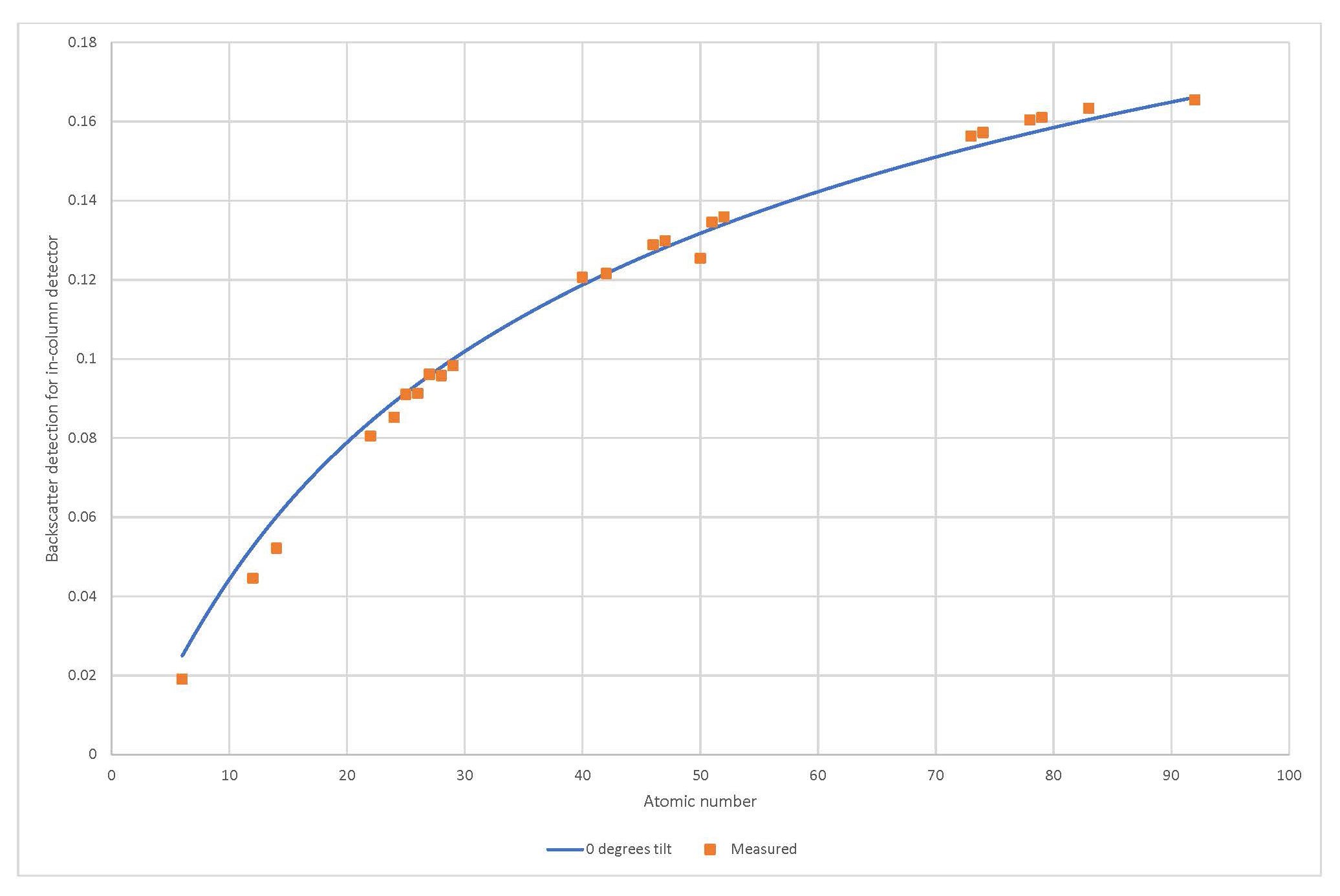 Figure 1 - Measured and calculated backscatter coefficients
Figure 1 - Measured and calculated backscatter coefficients
This property is often used in sample preparation by selecting a mounting material of a different atomic number than the particulate of interest to easily differentiate and outline the particles of interest.
It is easily overlooked that the backscatter coefficients are for flat surfaces perpendicular to the impinging electron beam and particles are not flat and surfaces are not always perpendicular to the impinging electron beam. (see figure 2)
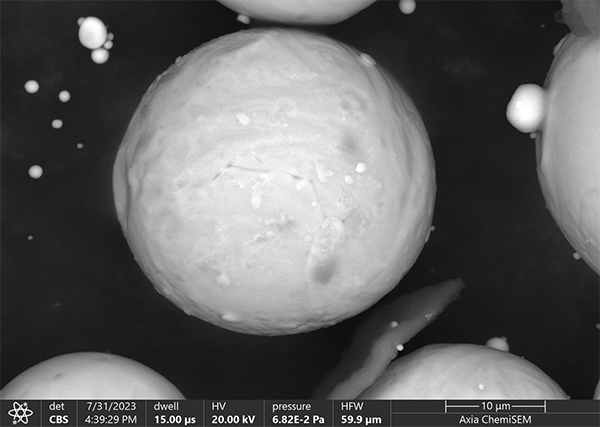 Figure 2 - Backscattered Electron image of Ti sphere on carbon background. Variations in grayscale of the central Ti sphere are due to different tilt angles with regards to the impinging electron beam
Figure 2 - Backscattered Electron image of Ti sphere on carbon background. Variations in grayscale of the central Ti sphere are due to different tilt angles with regards to the impinging electron beam
In this case the faces of the particle perpendicular to the impinging beam are brightest while the other surfaces of the particle are darker. Thus a homogeneous particle may exhibit various levels of gray depending on the angle of the reflecting surface. (see figure 3)
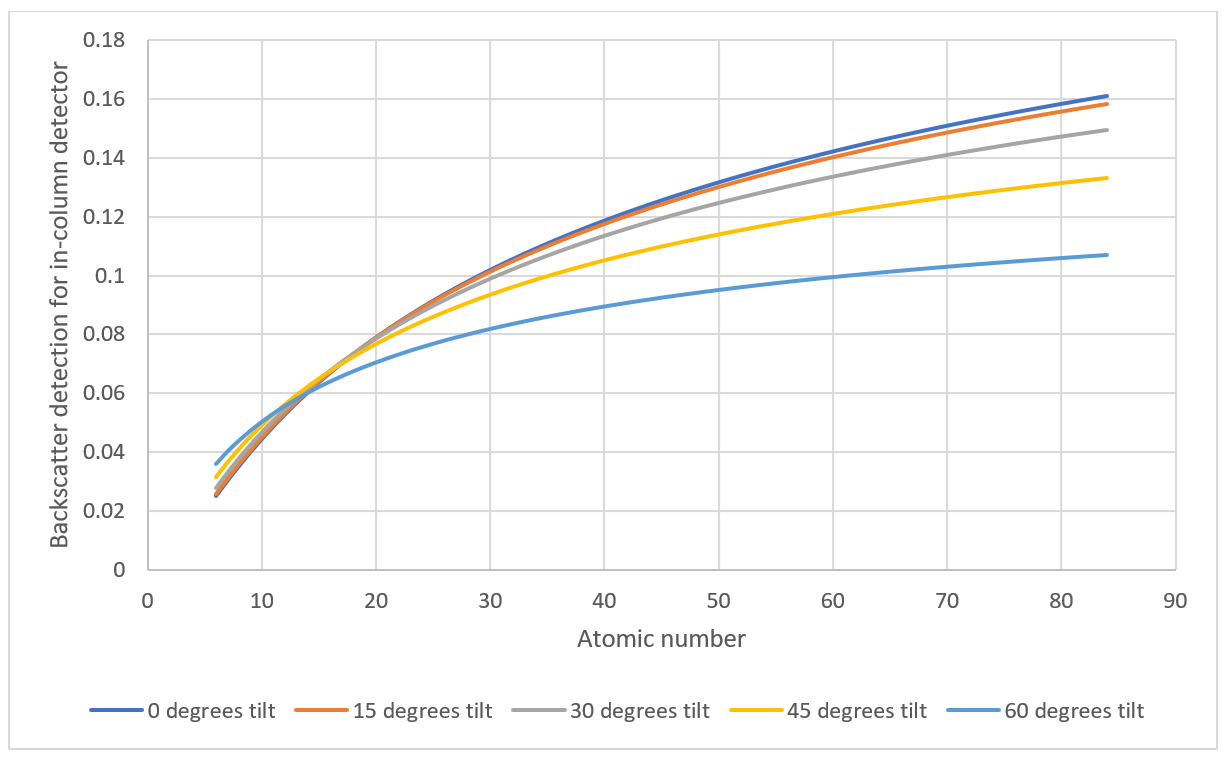 Figure 3 - Backscatter coefficients as a function of atomic number for various tilt angles
Figure 3 - Backscatter coefficients as a function of atomic number for various tilt angles
In conclusion, this application note highlights the relationship between backscatter coefficient, tilt angle, and atomic number. As the tilt angle increases, the backscatter coefficient becomes less dependent on the atomic number. This will be most pronounced for rough surfaces such as an agglomeration of various particles. For instance, at specific tilt angles, different materials like Fe, Sc, Co, Zn, and Ag exhibit similar backscatter efficiencies. (see Figure 4)
 Figure 4 - Screenshot of IntelliSEM identifying foreign contaminant in sample; identified with pin
Figure 4 - Screenshot of IntelliSEM identifying foreign contaminant in sample; identified with pin
Fortunately, most scanning electron microscopes are equipped with an energy-dispersive x-ray spectrometer, which can provide atomic concentration information near the point of impinging electrons. This feature proves valuable in confirming atomic concentrations when such details are obscured by surface structure in the backscattered electron signal.
For example, RJ Lee Group’s IntelliSEM® software was used to identify possible foreign particulate. (see figure 5) The presence of an Fe particle among Ti particles was confirmed with a Thermo Scientific Axia™ ChemiSEM™ Scanning Electron Microscope.
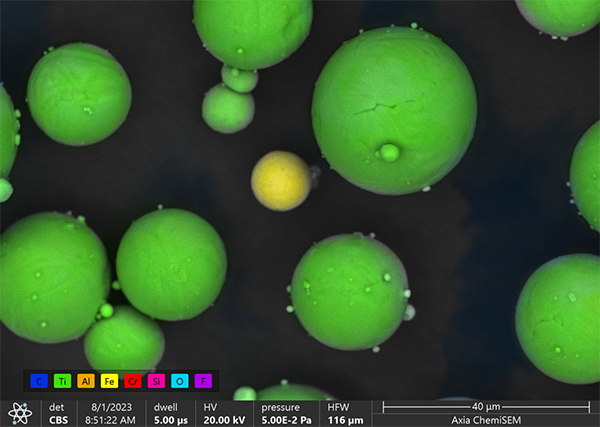 Figure 5 - zoomed-in detail of foreign contaminant as confirmed with ChemiSEM
Figure 5 - zoomed-in detail of foreign contaminant as confirmed with ChemiSEM
Share this post

